PDE/OEL Reports as per
EMA | PIC/S | APIC | WHO
Mutagenic & Genotox Assessments
as per ICH, M7
Comprehensive CRO Services for Pharma, Biotech & Medical Devices
Indivirtus delivers end-to-end pharmaceutical consulting and CRO solutions—from toxicological risk assessments and regulatory compliance to medical device registration and GMP approvals. Our expertise spans computer system validation, equipment and HVAC calibration, NABL-accredited calibration (thermal, electro-technical, mechanical, pressure, mass & volume), and turnkey greenfield/brownfield projects. We also offer specialized training in GxP, cleaning validation, and quality systems—helping you accelerate approvals, ensure compliance, and scale with confidence.
Indivirtus Healthcare Services
- Toxicological Services
- Pharmacovigilance
- Greenfield/ Brownfield Projects
- CSV and Calibration
- Regulatory Affairs
Indivirtus Global CRO
- Bioavailability and Bioequivalence (BA/BE) Studies
- Clinical Trials
- Bio-Analytical
- Medical Writing
Indivirtus Strategic Solutions
- Export/ import of finished formulations
- Finding a suitable MAH across all regions
Indivirtus AB7 Scribing and RCM
- Medical scribing
- Revenue cycle management
- Medical transcription
Indivirtus Solutions
- Insurance Services
- Accounting Services
- Data Management Services
- International B.P.O.
Indivirtus Ecological Services
- Environmental Risk Assessment
- Ecotoxicology Studies
- Biodiversity Compliance
Indivirtus Healthcare Services Pvt. Ltd.
is a globally recognized Contract Research Organization (CRO) delivering comprehensive, science-driven solutions to pharmaceutical and healthcare companies worldwide. We specialize in Advanced Toxicological Risk Assessment (including critical impurities analysis, genotoxicity evaluations, ADE/PDE/OEL/OEB reports), Expert Regulatory Affairs, NABL-accredited Precision Calibration Services, Comprehensive Pharmacovigilance, and end-to-end Greenfield/Brownfield turnkey project consulting. Our mission is to help pharmaceutical and healthcare organizations achieve seamless regulatory compliance, ensure unwavering patient safety, and accelerate successful product development and market entry across 45+ countries globally.
Comprehensive Service Offerings
Expert ADE, PDE, OEL, and OEB calculations for pharmaceutical cleaning validation that ensure patient safety and prevent cross-contamination in shared manufacturing facilities.
Our comprehensive ADE (Acceptable Daily Exposure), PDE (Permitted Daily Exposure), OEL (Occupational Exposure Limit), and OEB (Occupational Exposure Banding) calculation services provide the scientific foundation for effective cleaning validation protocols. We help pharmaceutical manufacturers establish safe exposure limits that protect both patients and workers from harmful API residues. Our toxicological assessments comply with EMA guidelines, ICH Q3D standards, ISPE best practices, and other leading international regulatory frameworks, ensuring your facility meets the highest safety and compliance standards.
Expert CRO services for comprehensive genotoxic impurity evaluation and medical device toxicological risk assessments. Ensuring regulatory compliance and patient safety.
Indivirtus delivers specialized Contract Research Organization (CRO) services for genotoxic impurity evaluation and comprehensive medical device toxicological risk assessment. Our experienced toxicologists follow internationally recognized regulatory standards including ICH Q3A(R2), Q3B(R2), Q3C(R8), and M7 guidelines for precise mutagenic impurity profiling, plus ISO 10993-17 standards for thorough evaluation of extractables, leachables, and their toxicological significance in medical devices. With over 3,200 successfully delivered Risk Assessment reports, our team of expert toxicologists provides evidence-based, data-driven risk assessments that ensure product safety and achieve seamless global regulatory compliance.

Comprehensive verification of isolator performance to ensure complete operator and environmental safety when handling highly potent compounds.
Our containment validation services ensure your pharmaceutical isolators provide maximum protection for operators and the environment when handling highly potent compounds (OEB 3+). We establish clear containment performance targets, conduct thorough surrogate powder testing, and perform comprehensive air, personal, and surface sampling at all critical control points. All samples undergo rigorous analysis at AIHA-accredited laboratories, with the entire process overseen by Certified Industrial Hygienists following strict ISPE SMEPAC guidelines and cGMP best practices for complete regulatory compliance.

Accurate and compliant preparation of Material Safety Data Sheets (MSDS) and Safety Data Sheets (SDS) for pharmaceutical compounds.
Our MSDS/SDS preparation service provides comprehensive safety documentation for active pharmaceutical ingredients (APIs), excipients, and formulations. These sheets are essential for safe handling, transportation, and regulatory compliance. We create GHS-compliant SDS that align with global standards including REACH, OSHA HCS, and CLP. Our service includes classification, hazard identification, toxicological data, and environmental considerations tailored for pharmaceutical settings.
Full-suite pharmacovigilance solutions from database setup to risk management plans for pharmaceutical safety monitoring.
Indivirtus provides end-to-end pharmacovigilance (PV) support as part of its CRO services for the pharmaceutical industry. From setting up PV systems and handling ICSR case processing to preparing PSMF, PSUR, and Risk Management Plans, our solutions are aligned with global regulatory standards (EMA, US FDA, WHO). Our team ensures your products meet all compliance requirements while protecting patient safety through vigilant signal detection, literature monitoring, and spontaneous reporting systems.

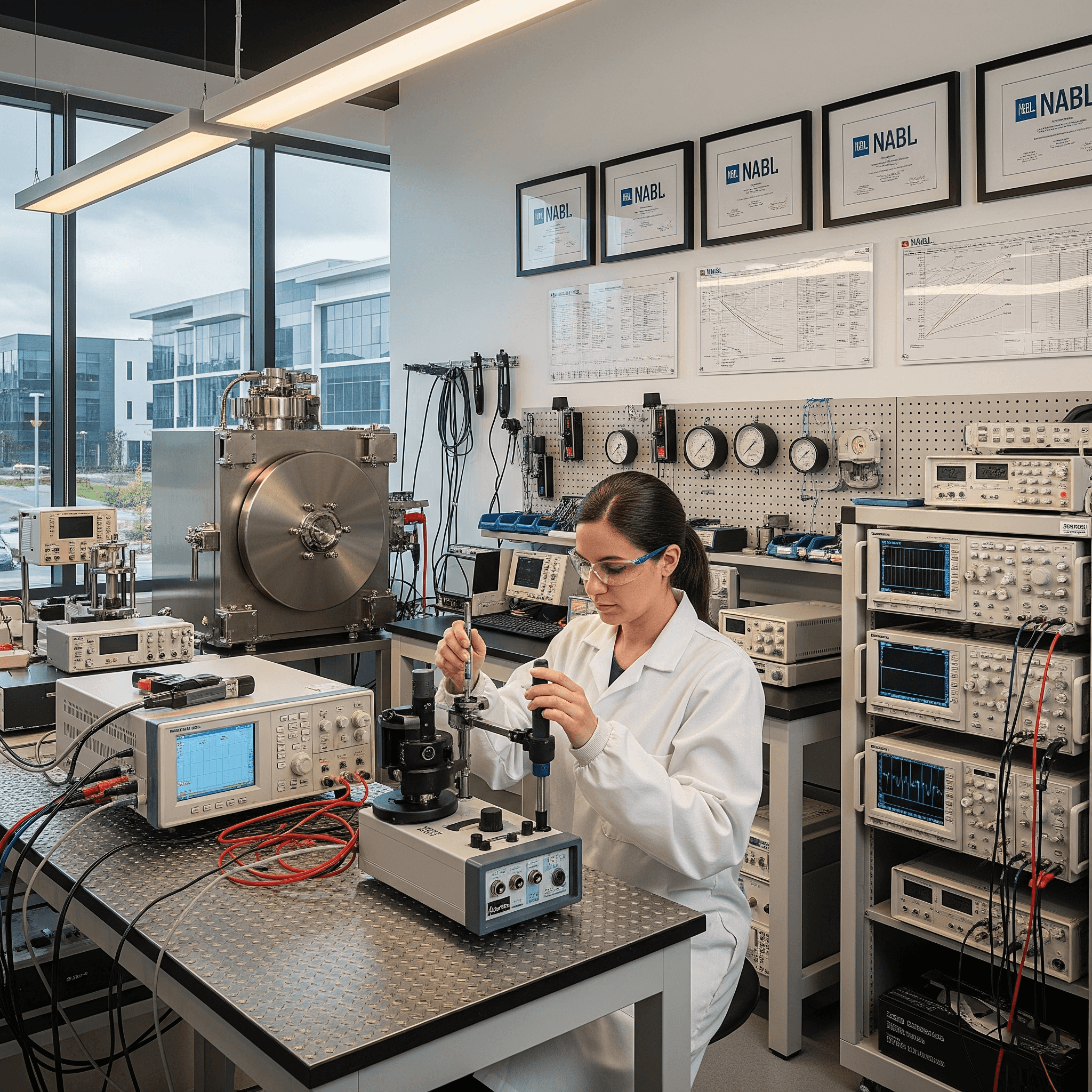
Keeping your critical instruments accurate and compliant with NABL-accredited calibration services you can trust.
Our NABL-accredited lab is your partner in precision. We provide complete calibration support to make sure your analytical, production, and utility instruments are always accurate and compliant. From temperature and humidity sensors to specialized meters for pressure, mass, and electrical readings, we've got you covered. Every calibration we perform follows traceable procedures that are respected by regulators worldwide.
Navigate global markets with ease. We offer expert guidance on CTD/ACTD dossiers, medical device submissions, CEP/DMF filings, and act as your official regulatory representative.
Getting your pharmaceutical or medical device to market shouldn't be a regulatory maze. Indivirtus offers clear strategic and technical guidance for your global submissions. We handle everything from compiling and submitting CTD/ACTD dossiers and DMF/CEP files to agencies like the FDA and Health Canada, to registering your medical devices under the latest MDR 2017 standards. Facing regulatory hurdles? We manage complex agency responses and remediation plans for you. We can also serve as your Marketing Authorization Holder (MAH) or Qualified Person (QP), and facilitate EU batch releases, ensuring your product not only complies with all regulations but also gets to market faster.

Hands‑on training on key topics like Cleaning Validation, GxP, Schedule M, WHO TRS, Data Integrity, Quality Culture, and QMS.
Indivirtus offers expert-led training programs tailored to pharmaceutical CRO teams and client staff. Our sessions cover essential topics: Cleaning Validation best practices, GxP standards (GLP, GMP, GCP, GDP), Revised Schedule M requirements, WHO TRS and annexures, creating a quality-minded culture, managing data integrity, and designing compliant quality management systems. Delivered by industry specialists, our programs include case studies, practical examples, and regulatory insight to build capability and compliance.

Comprehensive CRO support for GMP approvals from global authorities like EU-GMP, USFDA, WHO-GMP, TGA, and more.
Indivirtus offers end-to-end assistance for pharmaceutical facilities seeking GMP approvals from global regulatory bodies such as EU-GMP, USFDA, WHO-GMP, PIC/S, TGA, Health Canada, GCC, ANVISA (Brazil), and COFEPRIS (Mexico). Services include vendor assessment audits, plant design and infrastructure evaluations, validation and calibration checks, documentation reviews, and GAP assessments as per Schedule M and international GMP guidelines. Our experts help prepare for regulatory inspections with tailored remediation and compliance strategies.

End-to-end project execution for new (Greenfield) and upgrade (Brownfield) pharmaceutical manufacturing facilities.
Indivirtus offers turnkey consulting and implementation services for Greenfield and Brownfield pharmaceutical projects. Our CRO services include conceptual planning, design, execution, compliance, and technology transfer for Finished Dosage Forms (FDFs), Active Pharmaceutical Ingredients (APIs), and Intermediates. We ensure regulatory-ready facilities through a single-window approach—from layout to validation—meeting all global GMP requirements.

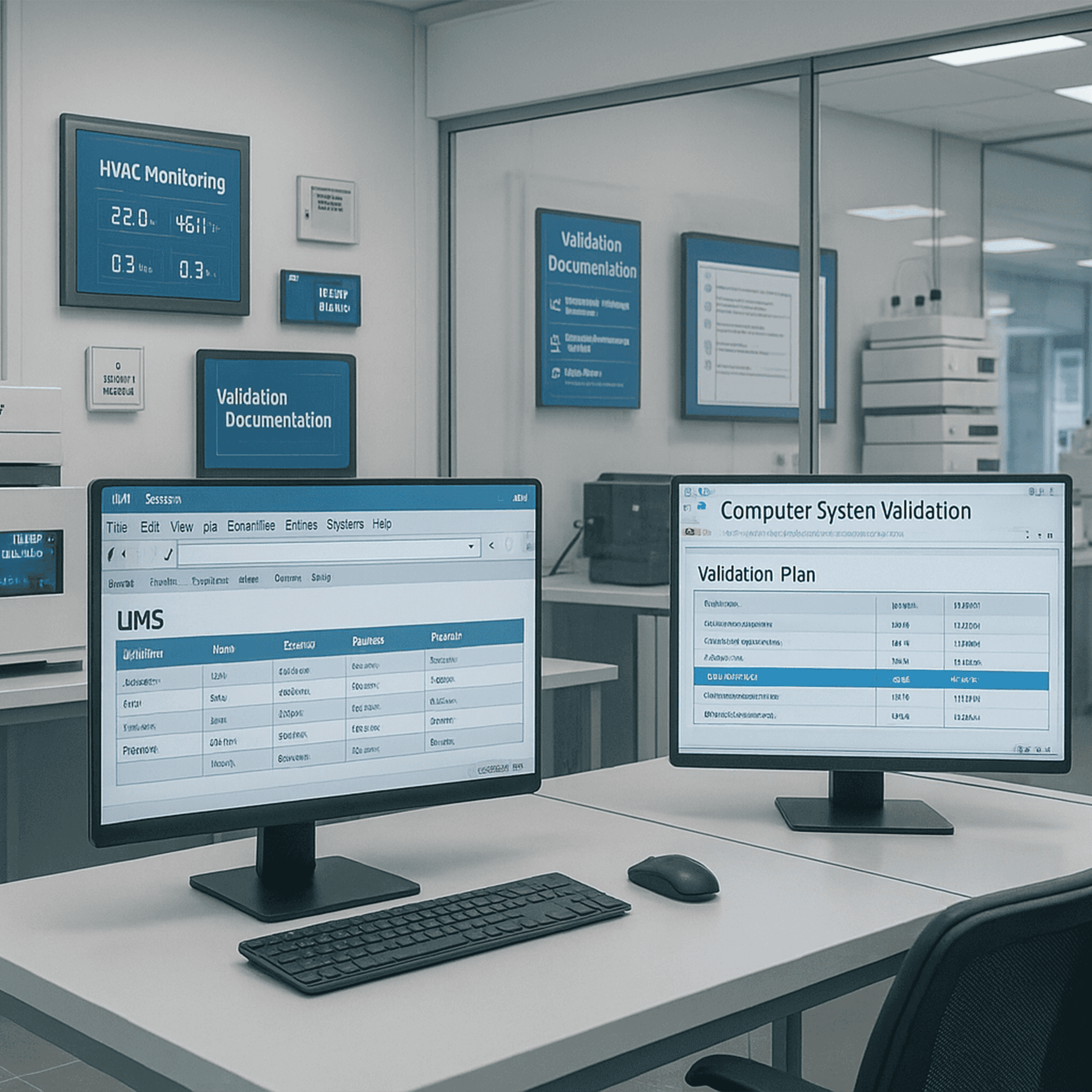
Validation of laboratory systems, process automation, EMS, LIMS, HVAC and temperature mapping to support regulatory compliance.
Indivirtus offers a full suite of validation and qualification services in support of pharmaceutical CRO operations. We validate computerized systems (CSV), laboratory instruments (HPLC, GC, FTIR, etc.), process automation platforms, environmental monitoring, enterprise software (LIMS, ERP, SAP), HVAC systems, and perform temperature mapping for critical zones. Our services ensure compliance with FDA, EMA, WHO and GMP standards, mitigating operational risk and ensuring data integrity.
Comprehensive GRAS evaluation services and CPSR preparation for FDA and EMA compliance in non-pharmaceutical industries, ensuring your products meet global safety standards.
Indivirtus provides expert regulatory compliance services for food additives, cosmetic ingredients, and consumer products. Our specialized team offers, FDA GRAS compliance, cosmetic safety report EU preparation, and comprehensive safety assessments to help your products successfully enter global markets while meeting stringent regulatory requirements.

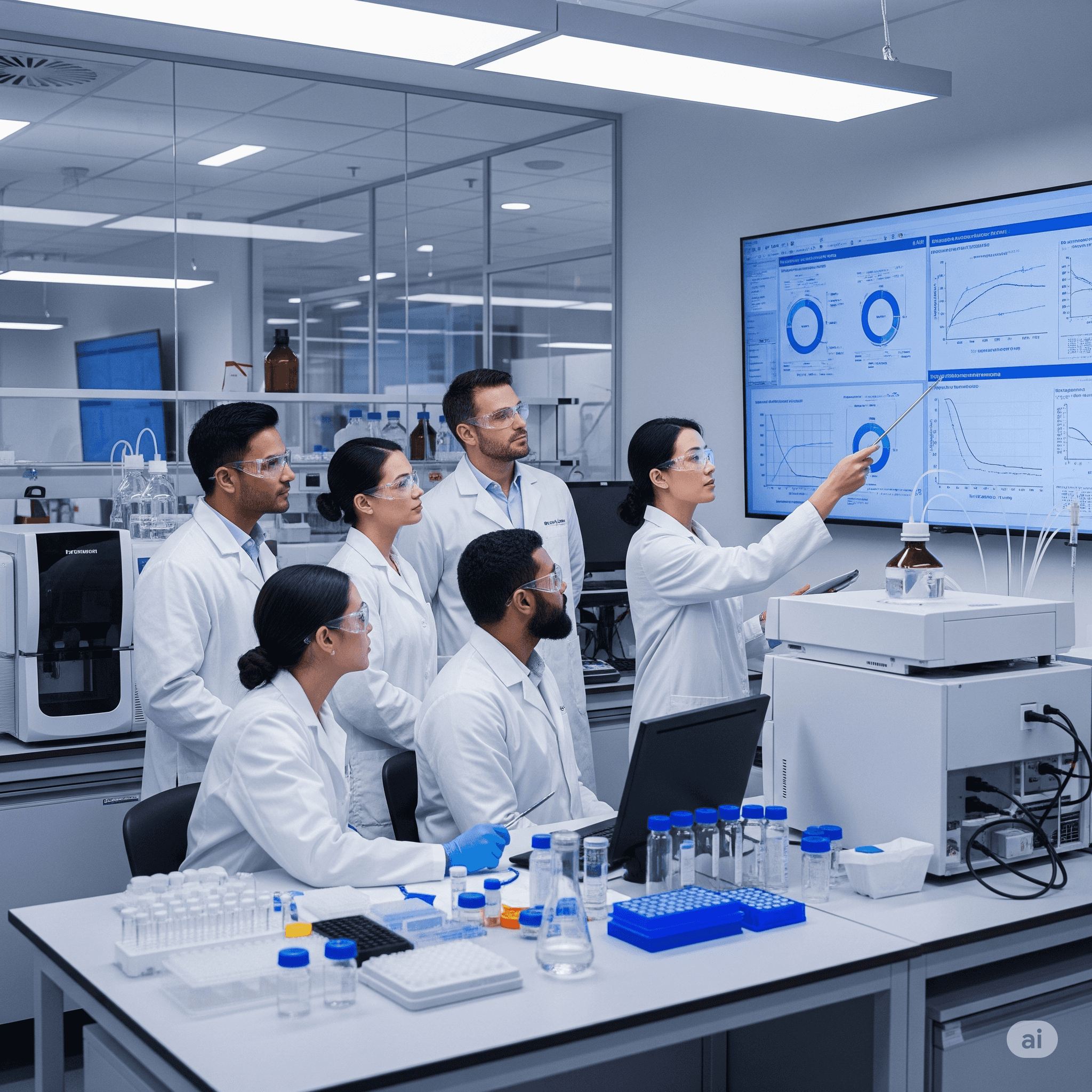
Independent QA oversight for BA/BE, pre-clinical, and clinical studies to ensure compliance, protect data integrity, and prepare for regulatory success.
At Indivirtus, we offer comprehensive QA support throughout your Bioanalytical, Pre-clinical, and Clinical studies. Our team helps you maintain GCP, GLP, and NDCT 2019 compliance while safeguarding scientific integrity, ensuring data reliability, and building confidence with global regulators.
Why Choose Us?
Regulatory Expertise
Deep understanding of USFDA, EMA, CDSCO regulations.
Certified Infrastructure
NABL certified labs, GMP-compliant facilities.
End-to-End Services
Complete solutions from preclinical to tech transfer.
Client-Centric Approach
Customized, transparent and responsive engagement.
Skilled Professionals
Qualified experts in science, QA, and compliance.
Data Integrity & Compliance
21 CFR Part 11, GLP/GMP practices ensured.
What our clients say!
“As per our experience in industry your service is one of the best, well trained people.”
Mayur Bhoir
Industry Professional
“Technically sound and professional services. In time delivery of reports at reasonable cost and complying global standards.”
Vicky Shinde
Professional Services
“Always timely support of documents with high quality assessment through knowledgeable SMEs including reasonable price.”
Subba Rao
SME / Quality Assessment
Our Global Footprint