What does CSV cover for process automation systems?
CSV includes risk assessment, requirement traceability, protocol design (IQ/OQ/PQ), execution, deviation handling and final report for PLC/HMI/SCADA systems.
How do you validate laboratory equipment like HPLC or FTIR?
We develop instrument-specific validation plans, conduct IQ/OQ/PQ runs, document performance qualifications, and assess calibration and maintenance needs.
What is temperature mapping and why is it necessary?
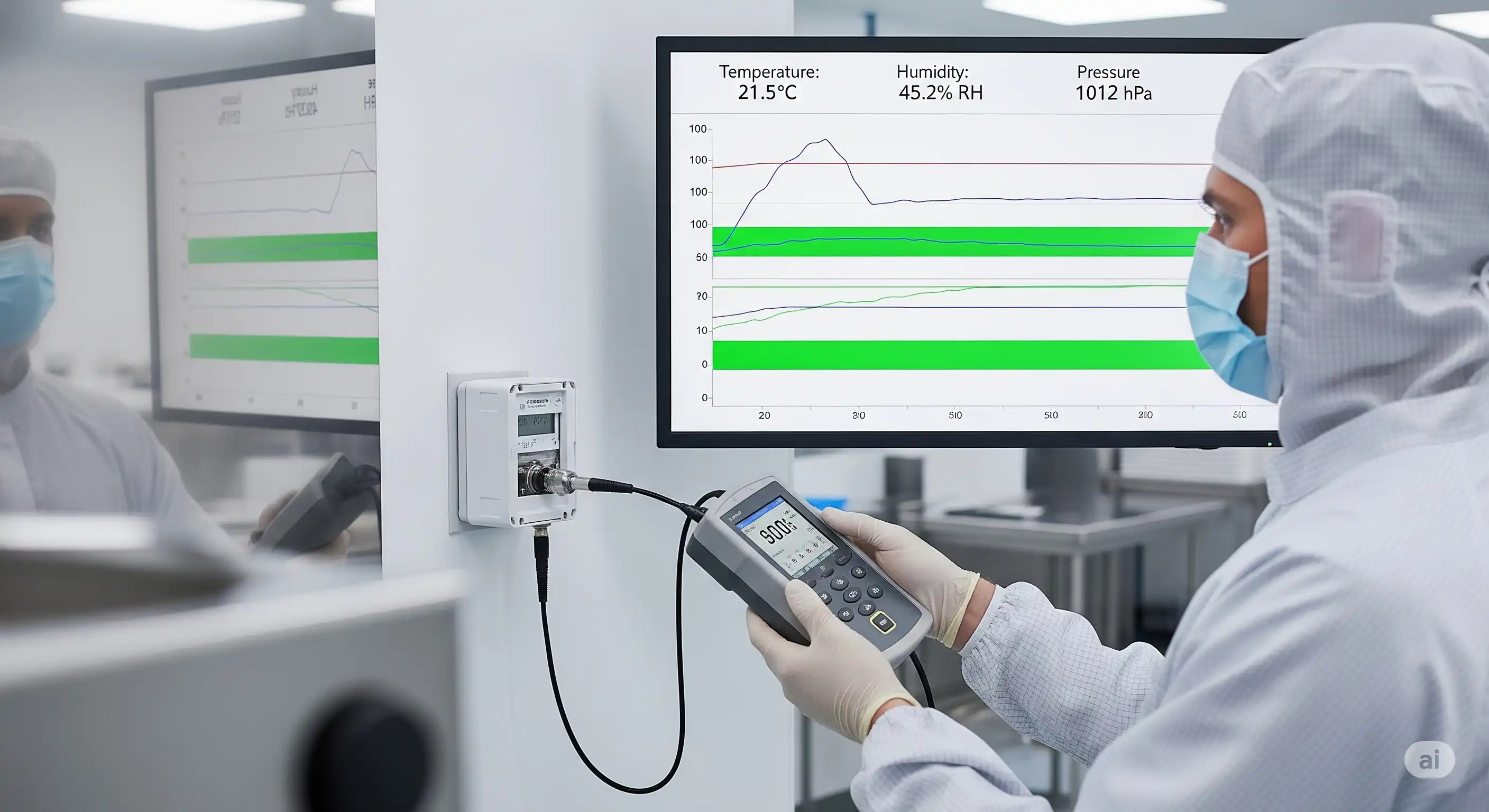
Temperature mapping documents spatial temperature uniformity and stability in critical environments (e.g., cold rooms, autoclaves) to ensure product safety and compliance.
What tests are included in HVAC qualification?
HVAC qualification includes DOP/fog testing, air velocity profiling, NVPC (non-viable particle counts), air changes per hour, and HEPA filter integrity checks.
Do you validate enterprise applications like LIMS or SAP?
Yes, we perform full CSV including requirement gathering, functional testing, user acceptance, and compliance to ensure systems meet GxP and 21 CFR Part 11 standards.
How long does a validation project typically take?
Validation timelines depend on system complexity and client readiness, but most projects complete in 2–8 weeks per system.
What is involved in laboratory systems validation?
Our laboratory systems validation includes DQ, IQ, OQ, PQ for instruments like HPLC, GC, and FTIR, along with analytical method validation, 21 CFR Part 11 compliance, and software validation for data acquisition systems.
How do you validate HVAC systems for cleanrooms?
We perform HVAC validation with DQ/IQ/OQ/PQ protocols, including airflow visualization, DOP/PAO testing, air velocity, particle counts, and compliance with ISO 14644-1/2 for cleanroom classifications.
What does temperature mapping entail?
Temperature mapping involves protocol development, pre/post-calibrated data loggers, and validation for autoclaves, tunnels, cold rooms, warehouses, and vehicles, ensuring data integrity and regulatory compliance.
How do you ensure compliance for enterprise applications like LIMS or ERP?
We execute CSV lifecycles (URS, FS/DS, IQ/OQ/PQ) for systems like LabWare, SAP, and Veeva, ensuring 21 CFR Part 11 compliance, risk assessments, and data integrity audits.
What industries benefit from your validation services?
Pharmaceutical manufacturers, biotech firms, CROs, and medical device companies benefit by ensuring compliant systems for quality, safety, and regulatory inspections.
What is the role of GAMP 5 in your validation process?
GAMP 5 provides a risk-based approach for validating automated systems like PLC, SCADA, and LIMS, ensuring compliance with regulatory standards and efficient validation processes.